Radioactivity
In this article, we are going to study about the phenomenon of radioactivity, which is related to some of the heavier elements.
 Table of Contents
Table of Contents- What is Radioactivity?
- Types of Radioactive Elements
- Types of Radioactive Decay
- Properties of Radioactive Rays
- Properties of Radioactive Decay
- Measurement of Radioactive Decay
- Applications of Radioactive Elements
What is Radioactivity?
Some elements (and their compounds) are not stable. They self-disintegrate by emitting some elementary particles or radiation (called radioactive rays). Such elements are called radioactive substances and this phenomenon is called radioactivity.
In other words, the nucleus of such radioactive elements is not stable and it tries to reach a stable state by emitting radioactive rays. This instability is due to the excess amount of neutrons in nuclei of atom.
So, radioactivity is caused by the nuclear instability of the atom. It’s a phenomenon related to the nucleus of an atom, i.e. it’s a nuclear phenomenon.
 Discovery of Radioactivity
Discovery of RadioactivityRadioactivity was accidentally discovered in 1896 by A. H. Becquerel, while he was studying the fluorescence and phosphorescence of compounds irradiated with visible light.
Later on, phenomenon of radioactivity was detected in thorium (and its compounds) by Madam Curie. She, along with her husband Pirre Curie, also found out that radium was radioactive too.
 Fluorescence and Phosphorescence
Fluorescence and PhosphorescenceFluorescence: Atoms of some substances absorb photons of higher frequency (i.e. shorter wavelength) and move to a higher energy state. However, these higher energy states are unstable and so soon they emit photons (i.e. light) of lower frequency (i.e. higher wavelength) and return back to their original state.
That’s why fluorescence substances emit light only in the presence of a light source. As soon as the light source (i.e. incident radiation) is cut-off, the emission of light by fluorescence substances stops.
This phenomenon of emission of light is called fluorescence, and such substances are called fluorescence substances.
Some examples of fluorescence substances are:
- Uranium oxide
- Barium platinocyanide – It is used in the search of X-rays.
- Tube light also makes use of this phenomenon.
Phosphorescence: This phenomenon is somewhat similar to fluorescence. However, phosphorescence substances can keep on emitting light even after the removal of the incident light.
Some examples of phosphorescence substances are:
- Zinc sulphide
- Calcium sulphide
- Barium sulphide
- Lamination of phosphorescent substances is often done on the needle of watches and in various hoarding boards (for marketing and ads). The phosphorescent substances on them absorb the sunlight in the day time and emit light in night.
Types of Radioactive Elements
There are two types of radioactive elements:
Natural Radioactive Elements: These are the radioactive elements that naturally exhibit the phenomenon of radioactivity. That is, they are found in nature. For example, Uranium (U92), Radium (Ra88), Thorium (Th90) etc.
Artificial Radioactive Elements: These are the radioactive elements that are not found in nature. Rather, these are synthetic radioactive elements – they are made in laboratories by artificial transmutation. All elements after Uranium have been prepared by artificial transmutation. For example, Np.
\(U^{238}_{92}\) + \(n^{1}_{0}\) → \({Np}^{239}_{93}\) + \(e^{0}_{-1}\)
Types of Radioactive Decay
We already know that Radioactivity is a nuclear phenomenon in which an unstable nucleus undergoes a decay. This is called as Radioactive Decay.
Radioactive Decay is of three types.
α-decay
Herein, an α-particle (i.e. a helium nucleus) is emitted by the nucleus.
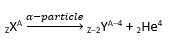
When this happens, the atomic number decreases by 2 and mass number decreases by 4.
β-decay
Herein, electrons or positrons are emitted by the nucleus. Positrons are the particles with the same mass as electrons, but with a charge exactly opposite to that of electron.
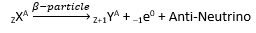
When this happens, the atomic number increases by 1 and mass number remains unchanged.
The nuclei that have high n:p ratio (i.e. that have excess neutrons) are found to decay by β emission.
γ-decay
Herein, γ–rays (i.e. high energy photons of hundreds of keV or more) are emitted by the nucleus.
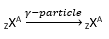
When this happens, the atomic number and mass number remain unchanged.
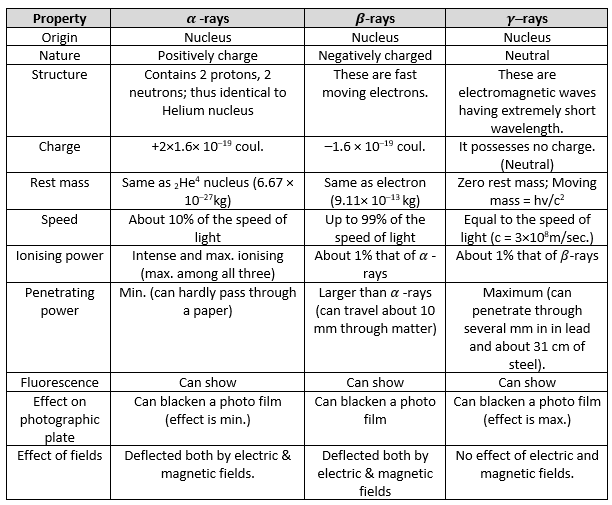
Properties of Radioactive Rays
Now, let’s have a look at the properties of radioactive rays, i.e. α, β and γ–rays.
Properties of Radioactive Decay
Radioactive elements do not emit α-particles and β-particles simultaneously. They will either emit α-particles or β-particles, but not both of them together at the same time. However, they may emit γ–rays along with α-and β-particles.
Also note that, the number of nuclei undergoing the decay per unit time in a given radioactive sample (be it any kind of decay - α, β or γ-decay) is proportional to the total number of nuclei in that sample.
There’s also a theory regarding radioactive decay. It’s called Rutherford-Soddy theory of radioactive disintegration. It states the following laws regarding radioactive decay:
- Radioactive decay is a random process. We cannot predict which atom will disintegrate first – it’s just a matter of chance.
- Radioactive decay is not dependent on physical conditions, e.g. pressure, temperature etc. That is, radioactive emission is a spontaneous process. We cannot speed it up, or slow it down by changing the physical conditions.
- Radioactive decay varies from one isotope to another, i.e. different isotopes of the same element may differ in their radioactive emission. So, radioactive decay is a characteristic of the isotope, and not an element.
Measurement of Radioactive Decay
We use certain concepts to measure radioactive decay, and compare radioactivity of various radioactive substances. These are given below.
Activity
It is not possible to measure the number of radioactive nuclei in a sample of radioactive substance. However, we can easily measure the decay rate of a sample. This is called the Activity of that radioactive substance.
There are a couple of very famous units that are used to measure the activity:
- The SI unit for activity is Becquerel (Bq). (named after Henry Becquerel, the discoverer of radioactivity). 1 Bq = 1 disintegration or decay per second.
- We also use Curie (Ci) to measure activity. 1 Ci = 3.7 × 1010 decays per second = 3.7 × 1010 Bq
- Another well-known unit is Rutherford. 1 rutherford = 106 decays per second = 106 Bq
Half Life
Half Life (T1/2) of a radioactive element is the time-period in which half the atoms of its sample decay.
 Note
NoteAverage Life (τ) = 1.44 × Half Life (T1/2).
Different radioactive atoms have different activity rates, i.e. they differ a lot in their rate of decay. Radioactive elements that have a half-life that is shorter than the age of the universe (15 billion years), have already decayed long ago. So, we do not find them in nature. They have to be produced artificially in nuclear reactions in our laboratories. Some examples of such short-lived radioactive elements are tritium, plutonium, etc.
Applications of Radioactive Elements
Now, let’s have a look at some of the use cases of radioactive substances.
Nuclear Bombs
One of the most famous use-case of radioactive elements is in nuclear bombs, e.g. fission bombs, fusion bombs.
Radioactive Dating
We also make use of the concept of radioactivity in gauging the age of objects, fossils, bones, geological events, etc. This age estimation is called radioactive dating, or radio isotope dating. Various naturally occurring radioactive isotopes are used for this purpose.
Radioactive elements decay over time. So, with time their amount will keep on decreasing over time in a sample. This helps us in predicting the age of that sample to a certain extent - be it rock, dead plants, organism or bio residue, etc. The technique of detecting the amount of a radio isotope in the sample for this purpose is called radioactive or radio isotope dating.
Carbon dating is the most common and famous form of radioactive dating. Prof. Libby of USA was the first one to suggest this idea. In it we make use of a radioactive carbon isotope, Carbon- 14.
Why is Carbon- 14 so useful in radioactive dating?
- It has a half-life of 5600 years, which makes it pretty useful for radioactive dating purposes.
- It is present abundantly in our atmosphere along with normal carbon. So, it allows us to find out the age of any organism or plant that intakes air, which has carbon-di-oxide.
But how is it done exactly?
- Carbon- 14 is incorporated into the atmospheric carbon dioxide molecules.
- When plants breathe CO2, they intake this radioactive carbon too.
- When herbivores animals eat plants, Carbon-14 enters inside them too.
With time the concentration of \(C^{14}_{6}\) in all living organisms reaches an equilibrium value of nearly 15 decays/minute. This remains stable till an organism is alive and breathing/eating.
However, as soon as an organism dies, it stops taking \(C^{14}_{6}\) from the atmosphere. This causes the concentration of \(C^{14}_{6}\) in the organism to decrease with time.
So, if we can measure the ratio of the concentration of radioactive carbon (\(C^{14}_{6}\)) to normal carbon (\(C^{12}_{6}\)) in any ancient organism, we can estimate the time when the organism died.
 Note
NoteThere are various kinds of elements and their isotopes present in our atmosphere. Most of these isotopes are stable, i.e. non-radioactive. However, when cosmic rays strike these isotopes, a number of radioactive isotopes are produced. Carbon-14 (\(C^{14}_{6}\)) is one such radioactive isotope. It is produced when atmospheric nitrogen is bombarded with high energy neutrons.
\(N^{14}_{7}\) + \(n^{1}_{0}\) → \(C^{14}_{6}\) + \(H^{1}_{1}\)
Obviously, radioactive carbon is unstable – a Carbon-14 atom decays by emitting β-particle, and produces nitrogen.
 Geological dating
Geological datingFor geological dating, i.e. to find the approximate age of rocks and minerals and even estimate the age of earth, scientists use radioactive uranium (\(U^{238}_{92}\)). This technique is called uranium dating.
 Neutron activation analysis
Neutron activation analysisNeutron activation analysis is a technique used for measuring the concentrations of elements present in very small amount. In this technique, sample is not destroyed.
Medical Uses
Radioactive elements are also used in medical science. One of the most well-known uses is that of cobalt-isotope (\(Co^{60}_{27}\)) being used for treating cancerous cells of tumors (by destroying them).
Some other medical uses of radioactive elements are:
- Re-59 is used to detect anemia. Radioactive iron is also used to detect diseases like anemia (and other malnutrition diseases), tuberculosis, etc.
- Radioactive sodium is used to locate the defect in blood circulations.
- Se-79 is used to detect disorders in pancreatic gland.
- Radioactive iodine in used to detect (and cure) the disorders in thyroid gland.
- P-32 (Radioactive phosphorus) is used for curing bone diseases and blood cancer (Leukemia). It is also used to study the uptake of and transportation of mineral salts (e.g. phosphorus) within plants.
Radioactive elements are used for numerous other purposes too. Let’s list down a few of them.
- Radioactive elements are used for tracing purposes. For example, in medicines to trace tumors in body, or to trace leakages in an underground pipe (carrying water or oils), etc.
- Radioactivity often leads to mutations in plants and seeds. This has led to development of new and improved plants.
- Scientists have even developed better and more effective insecticides with the help of radio-isotopes.
