Nuclei – Protons and Neutrons
In this article, we will focus on the nucleus of an atom, its constituents – protons and neutrons, and the force that keeps them together.
 Table of Contents
Table of Contents- What is a Nucleus?
- Protons and Atomic Number
- Neutrons
- Notation of Nuclides
- Stability of Nucleus
- Other Elementary Particles
What is a Nucleus?
Nucleus is at the center of an atom around which electrons revolve. It’s very compactly packed - volume of a nucleus is about 10-12 times the volume of the atom; however, more than 99.9% of the mass of the atom is contained within nucleus.
Two major constituents of a nucleus, i.e. the elementary particles, found inside a nucleus are protons and neutrons. Let’s study about these elementary particles in much more detail.
Protons and Atomic Number
Proton was discovered by Rutherford. In an experiment, when he bombarded nitrogen nuclei with α particles, protons were emitted and recorded. The concerned equation has been given below:
+ → +
Characteristics of Protons
- The charge on a proton is one unit of fundamental charge, i.e. + 1.6 x 10-19 coulomb. That is, it’s positively charged. In fact, nucleus of any given atom is positively charged because of protons only (as neutrons are neutral).
- The mass of a proton is 1.672 x 10-27 kg.
Atomic Number
We know that the negative charge on an electron (which are outside the nucleus) is the same in magnitude as the positive charge on a proton (which is inside the nucleus). Also, an atom is electrically neutral.
It means that total charge of the atomic electrons (–Ze) is equal to the total charge of the atomic protons in the nucleus of the atom (+Ze). It’s possible only if the number of electrons in an atom is equal to the number of protons in its nucleus.
Atomic number of an atom (Z) = Number of protons in the nucleus = Number of electrons in the atom if it’s electrically neutral
Neutrons
Scientists while studying isotopes proposed many hypotheses, one of which suggested that there may be some neutral particles in the nucleus that may be responsible for different atomic masses of isotopes. (We will study about Isotopes and Atomic Mass soon)
In 1932, James Chadwick verified this particular hypothesis. He bombarded beryllium nuclei with alpha-particles, and noticed that some neutral radiation was emitted.
+ → +
This neutral radiation was in fact neutron. He was awarded Nobel Prize in Physics in 1935 for his discovery of the neutron.
Characteristics of Neutrons
- Neutron is electrically neutral.
- The mass of a neutron is 1.6749 x 10-27 kg.
- Though neutron is stable inside a nucleus, a free neutron is unstable (In contrast, free proton is stable). A free neutron has a mean life of about 1000s and it decays into three elementary particles – a proton, an electron and an antineutrino.
Atomic Weight or Atomic Mass
Atomic Weight or Atomic Mass is the total number of protons and neutrons in a nucleus. As we often use a general term nucleon for a proton or a neutron, we can also say that Atomic Mass of an atom is the number of nucleons in its nucleus. It is represented by the letter A.
Another mathematical representation of atomic mass/weight is as follows:
Atomic weight = Weight of one atom of an element / One-twelfth of the weight of one atom of carbon (C-12)
Atomic Weight is also called Relative Atomic Mass. Mass spectrometer is the instrument we use to accurately measure atomic mass.
Measurement of the atomic masses of the atoms of the same element sometimes give different values. This revealed the existence of Isotopes.
Isotopes
Isotopes are the atomic species of the same element differing in mass. So, a single element can have various types of atoms that differ in mass.
All of them have the same number of protons (i.e. their atomic number is the same), and so all of them are placed at the same position on the periodic table of elements. In fact, Isotope is a Greek word that means “same place”.
Properties of Isotopes
- All the isotopes of a particular element have the same chemical properties. That’s because, chemical properties of elements depend on their electronic structure, and all isotopes have the same electronic structure (same number of protons, and so same number of electrons).
- However, physical properties of isotopes are different.
- Practically every element consists of a mixture of several isotopes. So, isotopes are pretty common. Even Hydrogen, which is the lightest element, has three isotopes.
 Isotopes of Hydrogen
Isotopes of HydrogenHydrogen element has three isotopes:
Hydrogen: It is the lightest atom of the three isotopes of hydrogen. That’s because in its nucleus there’s only one proton and no neutron. It is the most commonly found isotope (abundance of 99.985%).
Deuterium: As it’s an isotope of hydrogen, it also contains only one proton in the nucleus. But, apart from that it also contains one neutron in the nucleus. So, its atomic mass is double that of a normal hydrogen atom.
Tritium: As it’s an isotope of hydrogen, it also contains only one proton in the nucleus. But, apart from that it also contains two neutrons in the nucleus. So, its atomic mass is triple that of a normal hydrogen atom. Its nucleus is unstable and so it’s not found in nature. It’s produced artificially in laboratories.
 Note
NoteAmong all the elements of the periodic table, Polonium has the maximum number of isotopes.
 Isobars and Isotones
Isobars and IsotonesStudents often confuse among Isotopes, Isobars and Isotones. So, let’s understand these two terms too.
* Isobars: Isobars are the nuclides that have the same mass number (A) but different atomic numbers (Z). For example, , and are isobars. Similarly, , and are isobars.
* Isotones: Isotones are the nuclides that have the same neutron number, but different atomic numbers (Z) and mass numbers (A). For example, and ; and ; Phosphorus () and Silicon () are isotones.
Notation of Nuclides
So, we know that nuclei of various elements contain different protons and neutrons. Even nuclei of the same element may contain different number of neutrons.
To showcase the composition of a nucleus we use the following terms and symbols:
Z – It represents the atomic number, i.e. the number of protons.
N - It represents the neutron number, i.e. the number of neutrons.
A - It represents the mass number, i.e. the total number of protons and neutrons. So, A = Z + N.
Nuclear species or nuclides are shown by the notation , where X is the chemical symbol of the element.
For example, the chemical symbol of gold is Au. It contains 79 protons and 118 neutrons. It means it contains 79 + 18 = 197 nucleons. So, the nucleus of gold is denoted by .
Stability of Nucleus
We know that nucleus of atom is stable. But how so?
What force keeps the protons packed so closely together?
Why don’t the protons repel each other, as all of them have a positive charge?
Well, to know the answer to these questions we need to understand some concepts like mass-energy relation, mass defect, etc.
Mass defect, Nuclear Binding energy and Strong Nuclear force
When protons and neutrons come together to form a nucleus, some mass is lost. This phenomenon is called Mass Defect.
So, Mass Defect = Sum of the masses of protons and neutrons (that will eventually form the nucleus) in the free state – Mass of the nucleus
This missing mass, i.e. Mass Defect (∆m), is released in the form of energy (∆E) when the nucleus is formed.
As per Einstein’s mass energy equivalent relation, ∆E = ∆mc2, where c is the speed of light.
 Mass-Energy Relation
Mass-Energy RelationAs per Einstein’s theory of special relativity, mass and energy are equivalent, i.e. mass is another form of energy. We can convert mass into energy (say heat and kinetic energy), and vice-versa.
Einstein also gave the famous mass-energy equivalence relation, E = mc2. It’s perhaps the most famous equation in Physics.
Here, E is the energy equivalent of mass m, and c is the velocity of light in vacuum (approximately 3 × 108 m/s).
The released energy equivalent (∆E) is called Binding Energy of the nucleus, or Nuclear Binding energy. It is the energy that must be supplied to the nucleus to break it into its constituent particles. For average mass nuclei the binding energy per nucleon is approximately 8 MeV. This energy is much higher than the binding energy in atoms. This means that the force that keeps together a nucleus is very different from the force that keeps different atoms together. This force is called Strong Nuclear force, which is one of the four fundamental forces of nature.
In other words, Nuclear Binding energy is the energy required to break the Strong Nuclear force that binds the nucleus of an atom together.
Now, let’s have a look at some of the major properties of strong nuclear force.
- It is a strong attractive force that is strong enough to overcome the repulsion between the positively charged protons (and also neutrons), and pack them together into a tiny nuclear volume. Strong nuclear force is much stronger than the electromagnetic force (or coulomb force) that acts between charges – it has to be. Afterall, strong nuclear force has to dominate over the repulsive electromagnetic force that exists between protons in a tightly packed nucleus.
 Note
NoteThe gravitational force between masses is even weaker than the electromagnetic force (or coulomb force).
- Strong nuclear force remains strong only for very short distances. As the distance between two nucleons increases to more than a few femtometres, the magnitude of strong nuclear force decreases rapidly to zero.
- Strong nuclear force is independent of the electric charge on the particles. So, the nuclear force between neutron-neutron, proton-neutron and proton-proton will approximately be the same.
- We cannot represent strong nuclear force in any simple mathematical form (at least till now). (On the other hand, Coulomb’s law and Newton’s law of gravitation can be represented mathematically)
To summarize:
- Strong Nuclear Force and Nuclear Binding energy are the parameters of the stability of the nucleus. More the binding energy, more stable will be the nucleus, and vice-versa.
- It means that more the strong nucleus force, more will be the binding energy, and more will be the energy needed to break the nucleus.
- It means that less the strong nucleus force, less will be the binding energy, and less will be the energy needed to break the nucleus.
Other Elementary Particles
We have already studied about three elementary particles - electrons, protons, and neutrons. Now, let’s have a glance at a few other kinds of elementary particles.
Positron
It is the antiparticle of electron. It was the first anti-particle to be discovered. This was done by Anderson in 1932.
Positron is also called positive electron, because:
- Its mass is the same as that of an electron.
- It has a positive charge which is same in magnitude as that on an electron.
Antiproton
It is the antiparticle of proton.
- Its mass is the same as that of a proton.
- It has a negative charge which is same in magnitude as that on a proton.
- It has oppositely directed magnetic moment than that on a proton.
 Anti-particles
Anti-particlesThe existence of Anti-particles was predicted beforehand by Fermi-Dirac’s relativistic quantum theory of electron.
Both the particle and its anti-particle have:
- exactly the same mass
- exactly the same spin
- exactly the same life time (if they are unstable)
However, they have:
- opposite charge (if any)
- opposite alignment between the spin and magnetic moment
When a particle meets its anti-particle, they mutually annihilate (i.e. destroy each other producing immense energy).
Neutrino and Anti-Neutrino
Pauli discovered these fundamental particles in 1930.
- Neutrino and Anti-Neutrino are massless.
- Neutrino and Anti-Neutrino are chargeless.
- Anti-Neutrino is the antiparticle of neutrino. It has anti-spin.
Mesons
- Mass of mesons is intermediate between that of electron and proton.
- Mesons are of three kinds based on the charge they have on them: neutral, positive and negative.
- All mesons are unstable.
Quarks and Anti-Quarks
Quarks are elementary particles that are even more fundamental constituent of matter. In fact, quarks combine together to form other composite particles such as protons, neutrons.
Anti-Quark is the anti-particle of quark.
Bosons
Bosons are subatomic particles that have the following properties.
- Bosons have zero or integral spin.
- Mesons and Photons combine to form Boson.
 Note
NoteBosons have been named after Satyendra Nath Bose (an Indian Scientist).
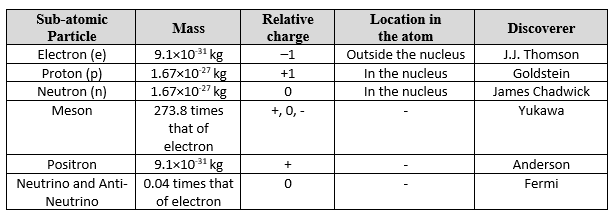
Now, let’s summarize the details of the various fundamental particles found in the atom.
 Cosmic rays
Cosmic raysCosmic rays are basically high energy atomic nuclei and fundamental particles continuously coming from deep space. In fact, every second around 1018 cosmic rays reach the surface of the earth.
The energy of these particles may range from 109 to 1018 electron-volts (so a wide range of energy). As they have high energy, these cosmic ray radiations are highly penetrating.