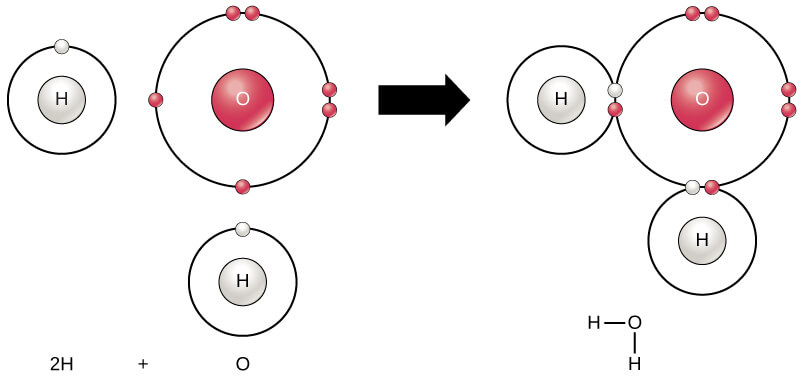
What are Atoms and Molecules?
In this article, we are going to learn about the concepts of atom and molecule.
 Table of Contents
Table of Contents- What is an Atom?
- What are Molecules?
- Concept of Valance Electrons
What is an Atom?
Every matter or element can be divided into smaller and smaller parts. Atom is the smallest part of a matter or element that showcases all the physical and chemical properties. If we divide an atom further, it will lose these basic properties.
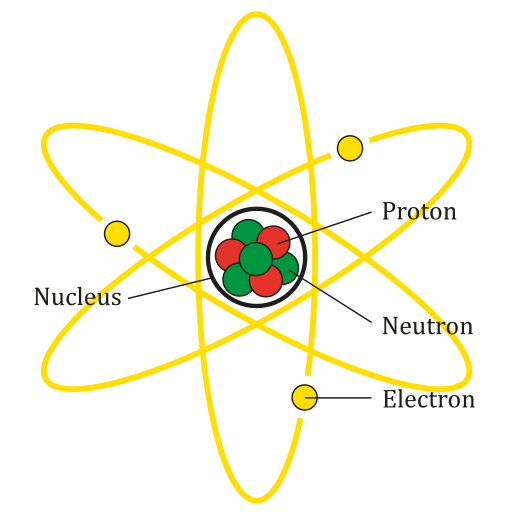
Though for a long-time philosophers and scientists thought atom to be the smallest part of matter. For example, as per John Dalton, atom was indivisible. But later experiments and observations proved that atoms are made up of smaller particles, i.e. atoms are divisible.
 Note
NoteIf you want to study the various models of the structure of an atom, you may read this article of ours.
What are Molecules?
Though atom is the smallest part of element that takes part in chemical reactions, but it does not exist in nature in free state. In nature we find molecules, which are made up of two or more atoms.
There are various kinds of Molecules that you will find in nature.
Atomicity of a Molecule
It is the number of atoms present in one molecule of a matter. For example:
- Diatomic molecules – Molecules having two atoms, e.g. of Hydrogen (H2), Nitrogen (N2), Oxygen (O2), etc.
- Triatomic molecules - Molecules having three atoms, e.g. Ozone (O3).
- Tetra-atomic molecules - Molecules having four atoms, e.g. Phosphorus (P4), Ammonia (NH3), etc.
- And so on.
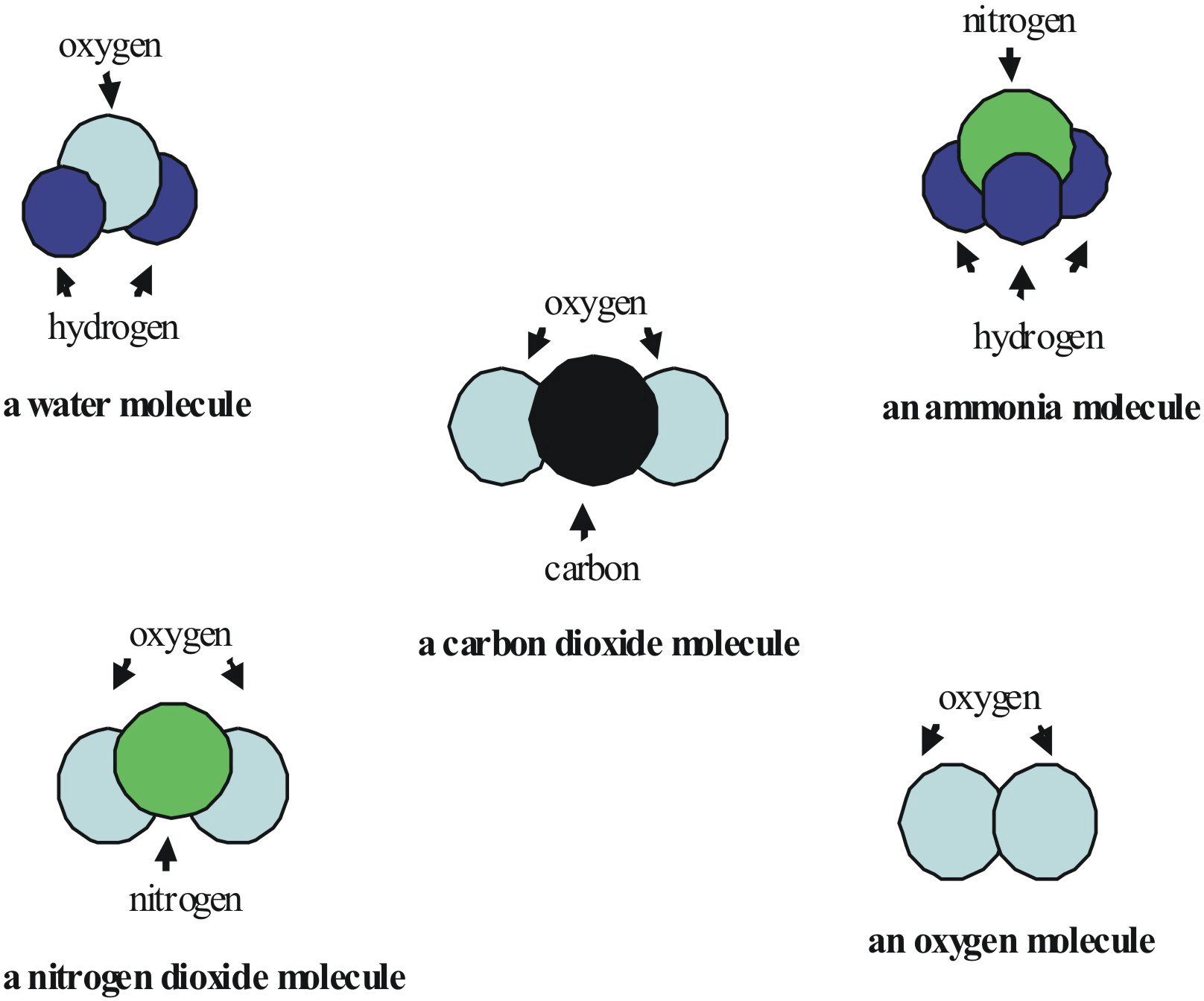
Homo-atomic and Hetro-atomic molecules
- Homo-atomic molecules have the same kind of atoms, e.g. H2, N2, O3, P4, etc.
- Hetro-atomic molecules have two or more different kinds of atoms, e.g. Ammonia (NH3) has one nitrogen and three hydrogen atoms; Water (H2O) has two hydrogen and one oxygen atoms, etc.
Concept of Valance Electrons
The electron or electrons that are present in the outermost orbit of an atom is/are called valance electron(s).
The number of valance electrons of an element is equal to the group number of the modern periodic table.
Only valence electrons take part in chemical reactions; not those residing in the inner orbits. That’s why elements having the same number of valence electrons have very similar chemical properties.